Host
- CHO cell-line
- Serum-free and adapted to suspension growth
Selection & USP
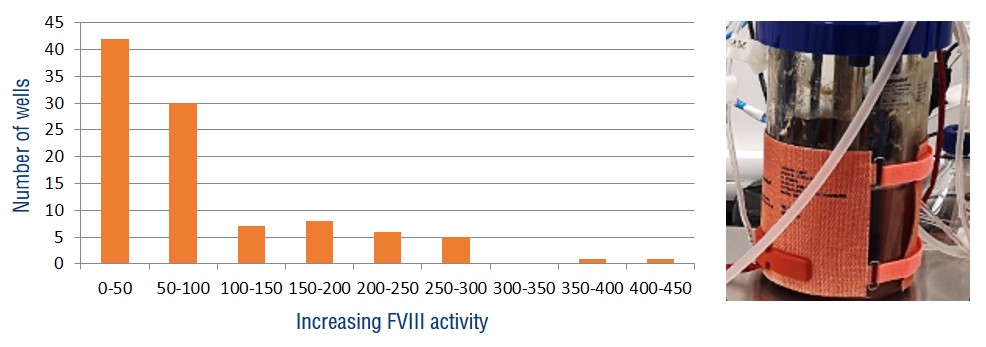
- Codon optimisation, chaperone-enhanced, clonal selection
- 3L scalable batch/perfusion process established

DSP
- 3-step chromatographic DSP
- Viral inactivation & removal (nanofiltration >85% recovery)

Pre-clinical material
- Fill and finish (outsourced)
- Freeze-drying (outsourced)
- Release assays
- Drug product completed pre-clinical toxicology